**Attention: We use cookies to help make this website better. By continuing to use our service, you agree to our use of cookies. More info**
**Attention: We use cookies to help make this website better. By continuing to use our service, you agree to our use of cookies. More info**
The best way to keep divers safe is to know the diving risks. An immediate obstacle to human activity under water is the fact that human lungs cannot naturally function in water environment. So, scuba diving is affected by two conditions: the pressure underwater and the air divers breathe.
Because water is denser than the air, while we descent the pressure will increase. This will create a problem for any air-filled spaces like the mouth, ears, para-nasal sinuses and lungs. This is because the air in those spaces is reduced in volume when under pressure.

At the beginning of the descent, an inability to equalize air pressure in the middle ear with outside water pressure can cause pain, and the tympanic membrane can rupture at depths under 3 m /10 ft. If not properly equalized.
To avoid this pain divers must equalize their ears while descending by pinching their nose and blowing really slowly through it. A practice of this exercise is recommended once before the dive and during the descent as many times as necessary.
When ascending or going up your ears and para-nasal sinus spaces will released the pressure automatically.
Being congested will increased the diving risks and can affect your ability to equalize your ears and release the pressure from ears and para-nasal spaces when ascending. You should avoid diving if when not feeling well.
While we descent the pressure increases and this affects the gases by decreasing the volume of them. See examples 1 and 2. Example 1 represents a bottle at the surface and example 2 a bottle at 20m / 66ft with 3 Bar/ 3 Ata of pressure. While we ascent the opposite happens, bringing the bottle back to its normal size.
But diver's lungs are not like the bottle because we are breathing underwater through the scuba gear. So, what really is affected are the volume and the density of the gases in our body.
Now imagine that we put air in that bottle at a depth of 20m / 66 ft and start ascending. The pressure will now decrease and the volume will increase creating a pressure inside the bottle that may make the bottle explode at the surface. This is the reason why to prevent diving risks, divers with scuba gear never hold their breath while scuba diving, making this the first rule of scuba diving - Always Breathe, never hold your breath. And due to this reason divers when ascending always do so very slowly. Take as a reference your computer or the little bubbles you are exhaling and never go faster than the smallest ones.

Example 1 (at the Surface)

Example 2 (at depth of 20m / 66ft)
Our latest post to keep you updated.
 Best Diving Cameras.
Best Diving Cameras.
These are currently the best cameras for diving.
 Travelling with Diving equipment
Travelling with Diving equipment
The airlines are in the endless battle to reduce their costs...
 Amed-Bali.
Amed-Bali.
most commonly known for diving the USS Liberty wreck at Tulamben area.
Ria Aldan.
Part of the Rias Baixas in Spain.
 Khao Lak Beach.
Khao Lak Beach.
Located in Thailand with over 20+ nice dive sites.
Diving Karon.
Located in Thailand with over 30 nice dive sites.
 Diving in Canada.
Diving in Canada.
The water is cold, but that has not stopped the Canadian divers and visitors.
 Diving in Exuma, The Bahamas.
Diving in Exuma, The Bahamas.
with 365 cays and islands is the perfect place for relaxation and diving.
 The Bahamas
The Bahamas
known for its big tourism industry offering big hotels, nice beaches and clear waters.
 Diving in Kalymnos, Greece.
Diving in Kalymnos, Greece.
Diving in Kalymnos Island, Greece. known as the "Sponge-divers' island.
Diving in Paphos, Cyprus.
Paphos is one of the oldest cities in the world which has seen rise and fall of many kingdoms.
 Diving Raja Ampat, Indonesia.
Diving Raja Ampat, Indonesia.
Famous for its ditch biodiversity and the coral coverage is stunning.
Diving Aliwal Shoal-Reef-Umkomaas, South Africa.
The most popular dive site to view the Ragged Tooth Sharks during the shark season.
 Diving in South Africa.
Diving in South Africa.
Cage diving in South Africa is very famous due to the massive fur seal colony and African penguins.
Diving Kata Beach-Phuket, Thailand.
Kata Beach in Phuket has most beautiful beaches and easy to dive locations on the island.
Diving Santa Catalina, Panama.
Most of our dive sites are located in Coiba National Park, a protected area.
 Diving in Bali.
Diving in Bali.
Bali has lots of great dive and snorkelling sites.
 Diving Carriacou, Grenadine Isl.
Diving Carriacou, Grenadine Isl.
Carriacou prides itself on some of the best diving in the Caribbean.
 Diving Guardalavaca
Diving Guardalavaca
Guardalavaca was a nice experience. They have a great wall with really nice tunnels.
 How is the coral reef being affected?
How is the coral reef being affected?
Corals are highly sensitive to environmental changes that can kill them.
 Diving Kas-Turkey
Diving Kas-Turkey
Kas is a small historical town on the south coast of Turkey and we have great diving.
Diving South Dalmatia
Diving Marco Polo's home town in Croatia.
 Catalina Island, Dom Rep
Catalina Island, Dom Rep
You will love our amazing snorkelling and diving activities.
What we are trying to avoid by breathing normally and continuously while slowly ascending is an air embolism (gas or air bubbles in our vascular system), which is a more severe hazard at shallow depths if a diver ascends as little as three feet without venting the expanding gas volume in the lungs.
We will explain why. At 10 m / 33 ft below the surface the pressure is double (2 Bar / 2 Atmospheres) the pressure at the surface. This will decrease the volume of gases by haft (1/2) and at 20m / 66 ft (3 Bar / 3 Ata) will decrease the volume of the gases only by one third (1/3). Making the ascent from 10m /33 ft to the surface the most dangerous phase of the ascent.
The density underwater is also effected the same way as pressure. Pressure increases as density increases, requiring more of the molecules of the gases to fill the same space.
| Depth | Pressure | Gas Volume | Desity |
|---|---|---|---|
| 0 | 1ATM/BAR | 1 | x1 |
| 10M/33F | 2ATM/BAR | 1/2 | x2 |
| 20M/66F | 3ATM/BAR | 1/3 | x3 |
| 30M/99F | 4ATM/BAR | 1/4 | x4 |
| 40M/131F | 5ATM/BAR | 1/5 | x5 |
Why I breath more the deeper I go? It is all on how the pressure affects the gasses you breathe. See the next table: the deeper you go the pressure and density increasses, but the volume of gasses will decrease. Making you need more air on every breath you take.
For this reason divers need twice the air at 2 Bars / 2 Ata, at 3 Bars / 3 Ata divers will breathe three times the air at the surface. The deeper we go the more gases we use, increasing the diving risks.
Exact calculations or predictions on how long an individual's dive can last are impossible due to the factors like cold, stress, fitness and experience.
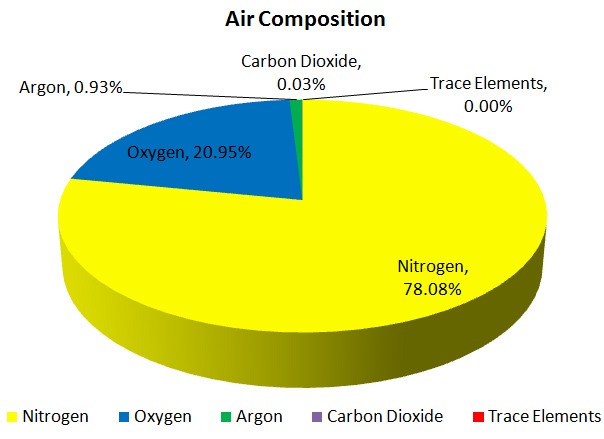
A lot of people think, that the gas divers breathe while scuba diving is oxygen. Although the human body needs oxygen to survive, under pressure oxygen can be toxic. So, the gas divers use from the tank is compressed normal air.
Air is composed of 78.084% of nitrogen, 20.946% of oxygen and 1% of other gases that have little or no effect when breathing compressed air. That's the reason a lot of instructors explain the composition of the air as 79% nitrogen and 21% of oxygen.
Nitrogen is the most abundant gas in the atmosphere, but is not used by humans during respiration. This gas can cause serious issues while diving and increase the diving risks.
Nitrogen under high pressure can temporally effect our nervous system and interfere with signal transmissions, causing at greater depths (30 to 40 meters/100 to 133 feet) the condition known as nitrogen narcosis. The effect is similar as being under the influence of alcohol (loss of decision-making ability, loss of focus, impaired judgment, multi tasking and coordination).
The most straightforward way to avoid nitrogen narcosis and create further diving risks is for a diver to limit the depth of dives. If narcosis does occur, the effects disappear almost immediately upon ascending to a shallower depth.
addition to its narcotic effects, nitrogen also brings another issue that adds to the diving risks. We mentioned already that our body does not use nitrogen during the respiration while scuba diving. Under pressure nitrogen dissolves into body tissues and starts to accumulate. This must be kept within limits to prevent nitrogen from coming out of solution and forming bubbles inside our body, known as "decompression sickness" or "the bends".
Pain and skin rash frequently in the limbs and joints, is the most common symptom of decompression sickness. Numbness, dizziness, weakness and fatigue are also very common symptoms to watch for.
The primary first aid for decompression sickness is to administer oxygen and lie the patient on his left side. Rapid decompression treatment in a chamber has proven highly effective in reducing or preventing permanent injuries.
To avoid the bends divers must minimize the water pressure on the body slowly at the end of each dive. This will allow the gases trapped in the bloodstream to gradually break solution and leave the body. This is done by ascending slowly and making safety stops or decompression stops using dive computers or decompression tables for guidance.
Knowing the time limits for each depth will avoid decompression sickness" or "the bends or other diving risks.


The use of diving computers and tables will keep you save and will help avoid diving risks.
you can see while scuba diving, dives are limited in time and depth due to the nitrogen. Today thanks to the new technology we have managed to extend our limits. For those divers that exceed 40 meters/132 feet and for divers who need to spend a lot of time under water, a different gas mixture, training and equipment are required.

Technical dive is when divers carry more than one cylinder, containing different gas mixtures for a distinct phase of the dive (descent, bottom, and decompression). These different gas mixtures may be used to extend bottom time, reduce inert gas narcotic effects, and reduce decompression times.
The most commonly used mixture is Enriched Air Nitrox, which is air with extra oxygen, often with 32% or 36% oxygen (called EAN32 and EAN36, or Nitrox32 and Nitrox36, or Nitrox I and Nitrox II). This mixture, of course, with less nitrogen, will reduce diving risks and more important the decompression sickness. Due to the oxygen toxicity divers use nitrox only in dives where they need to spend a lot of time in depth less than 40 meters/ 132 feet, and also during the beginning of their descent to deep (or deeper) dives.
A mixture of oxygen with helium that also reduces the percentage of nitrogen is known as trimix. The lower density of helium reduces breathing resistance at depth allowing the gas mix to breathe safely on deep dives. This mixture is often used during the deep phase of technical diving and in deep commercial diving. For example, a mix named "trimix 10/70" consisting of 10% oxygen, 70% helium,
20% nitrogen is suitable for a 100 meters (330 feet) dive, but it cannot be safely used at shallow depths. Heliox, a mixture of 21% oxygen and 79% of helium is also used for deep dives.
Because sound travels faster in Helium than in air, during the deep dive, divers with communication systems have very high-pitch voices, which may be hard to understand to people not used to it.
Remember that being underwater has limits and risks that professional divers are willing to take. It is important to remember that recreational scuba diving is for fun. Dives between 5 to 20 meters/ 16 to 66 feet can show you the wonderful world that was once explored by Cousteau. These depths have the advantage that provide divers with better light, colours and marine life. Also during shallow dives you will breathe less air from the tank, making your dive longer and safer.
For more on
Safe Scuba Diving Press Here
If you will like to add to more to "Diving Risks"
Press Here


Best Diving Cameras | Diving Information

Top 10 Diving Accessories | Diving Equipment

Russian Tanks in Cuba | Diving Video
The Coral Reefs | Diving Information
Afraid of Diving | Diving Information

12 Common Diving Mistakes | Diving Information

Necessary diving equipment | Diving Equipment

First Timers | Diving Information
Diving Signals | Diving Information
Diving Risks | Diving Information

Equipment Maintenance | Diving Equipment

Diving History | Diving Information
Diving Questions | Diving Information

Diving Quotes | Diving Gallery

How deep is 3 bars?| Diving Information
See More.
How to become a contributor